Analytical Quality Assurance – Rethought
How can we maximize confidence in analytical outcomes — whether derived from physical, chemical, biological or digital tests — without disproportionate resource use?
QuoData Solutions combines scientific depth with practical innovation that harnesses mathematical-statistics and predictive analytics. We enhance regulatory bodies' validation and verification capabilities, solidify laboratories' procedural trustworthiness, and strengthen companies' confidence in their return on investments.
From Traditional Validation to Predictive Quality and Smart Quality Monitoring
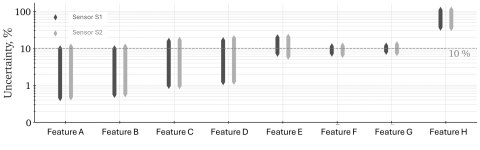
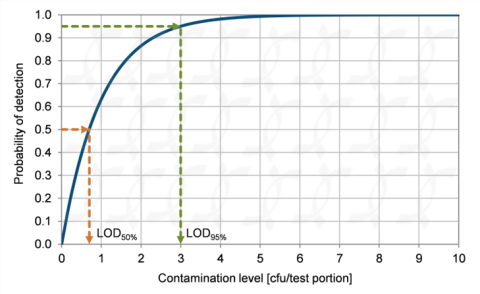
Whether proficiency testing, method validation, or uncertainty analysis — our tools facilitate data-driven decision-making at the highest level. With AI-driven decision support, we assist key stakeholders in industry and regulatory bodies in securely and robustly implementing AI-supported analytical processes.
Global Presence – Locally Rooted, Globally Connected
We support laboratories, public authorities, and businesses worldwide with practical, scientifically robust tools for analytical quality assurance. Our software solutions and services not only enable traditional validation and evaluation but also pave the way to predictive quality, automated result evaluation, and smart quality monitoring.
Whether in food safety, environmental analysis, or other research, QuoData stands for informed decision-making based on intelligent data analysis.
QuoData is your reliable partner for process, method, and device development as well as lab assessment.
QuoData solutions for different industries
Get to know QuoData’s mission and core team members
At QuoData, you can shape the future of quality assurance, working alongside enthusiastic statisticians, software developers, and AI pioneers.
Used in over 30 ISO 17043-accredited proficiency testing programs
QuoData services and tools are used worldwide by ISO 17043-accredited PT providers.

QuoData's scientific papers for the advancement of quality assurance
Erfolgsgeschichten unserer Kunden
Our unwavering commitment to client satisfaction is evident in the countless testimonials we've received from satisfied customers.